Hlongmed
ABOUT US
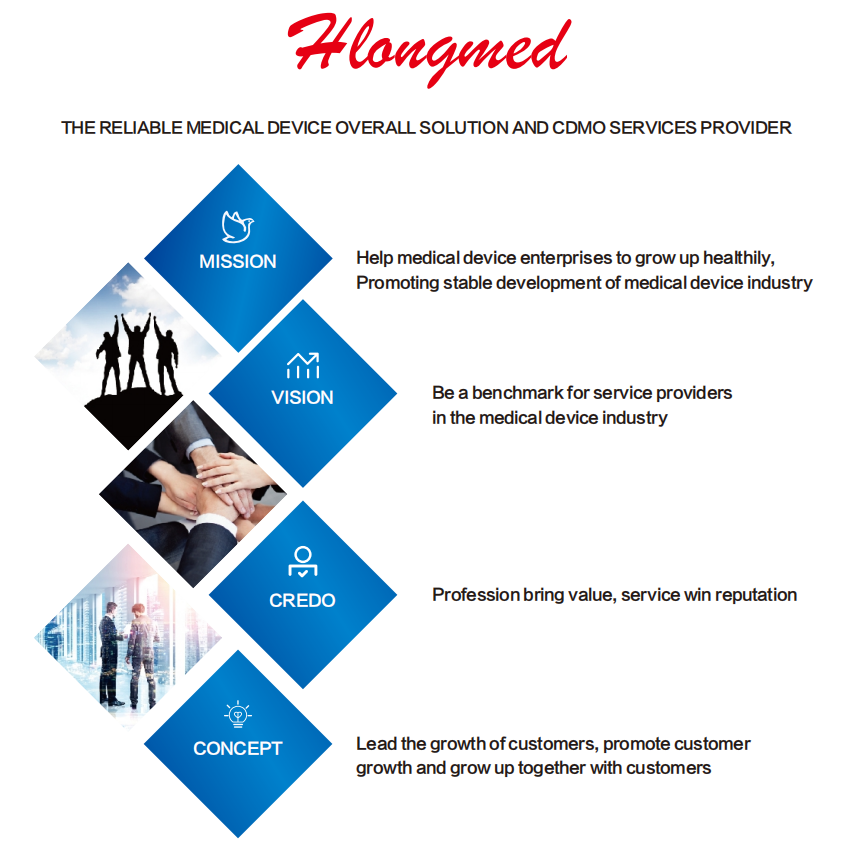
ABOUT US
Hlongmed
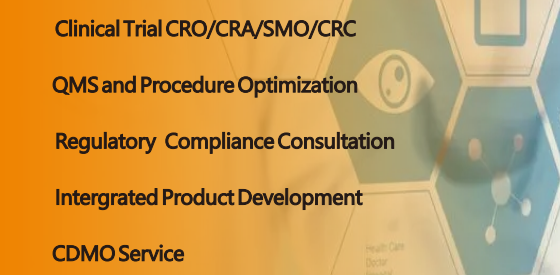
Hlongmed ® is one of the Professional leaders of Medical Device Regulatory Partner, CDMO,CRO/CRA/SMO/CRC Partner, and The Reliable Medical Device Overall Solution and CDMO Services Provider.
Hlongmed ® has subsidiaries or Branch in USA, Ireland, Beijing, Wuhan, Suzhou, Changsha, Chengdu andChongqing and one Institute in Shenzhen. Over 3000 domestic and international medical device enterprises,Authorized parties, hospitals, NB and NGO have entrusted Hlongmed ® services.
With rich Experiences in Clinical trial, CDMO, regulatory compliance and procedure excellence, Hlongmed ® team have established Long-term friendly relationship with clinical trial sites, NB, competent Authorities, TestLab, Associations, Investor, IP and Law servicers.
|
|